EAGLE IS MORE THAN TESTING, LET OUR KNOWLEDGE WORK FOR YOU
GAP ANALYSIS AUDIT | COMPLIANCE SOLUTIONS & CONSULTING | CALIBRATIONS & CERTIFICATIONS | TESTING | & MORE
Shield Analysis Process Control Charts
Process Control Charts – Harness the Power of Trending
The new Shield Analysis feature of EagleTrax provides a comprehensive and retrospective review of personnel and environmental monitoring data as well as critical quality attributes of the compounded preparation, enabling users to analyze trends over time. By examining data points collectively, Shield Analysis can assist in interpreting the overall state of microbial and product quality control. The process control charts that are generated through Shield Analysis may serve various purposes, including but not limited to the proactive identification of adverse trends, review of data through graphical representation, monitoring of microbial flora and evaluation of seasonal trends, evaluation of the cleaning and disinfection program, satisfy regulatory requirements for trending and annual product review, and identification of problem areas.
Thank you for reading this post, don't forget to subscribe!Process Control Chart Types
We offer various types of reports, including Microbial Identification, Potency Average, Potency Multiple Analytes, Potency Trends, SCANRDI®, USP <71> Sterility, USP <85> Bacterial Endotoxin, and USP <800> Hazardous Drugs reporting. Here are some examples of reports for your reference.
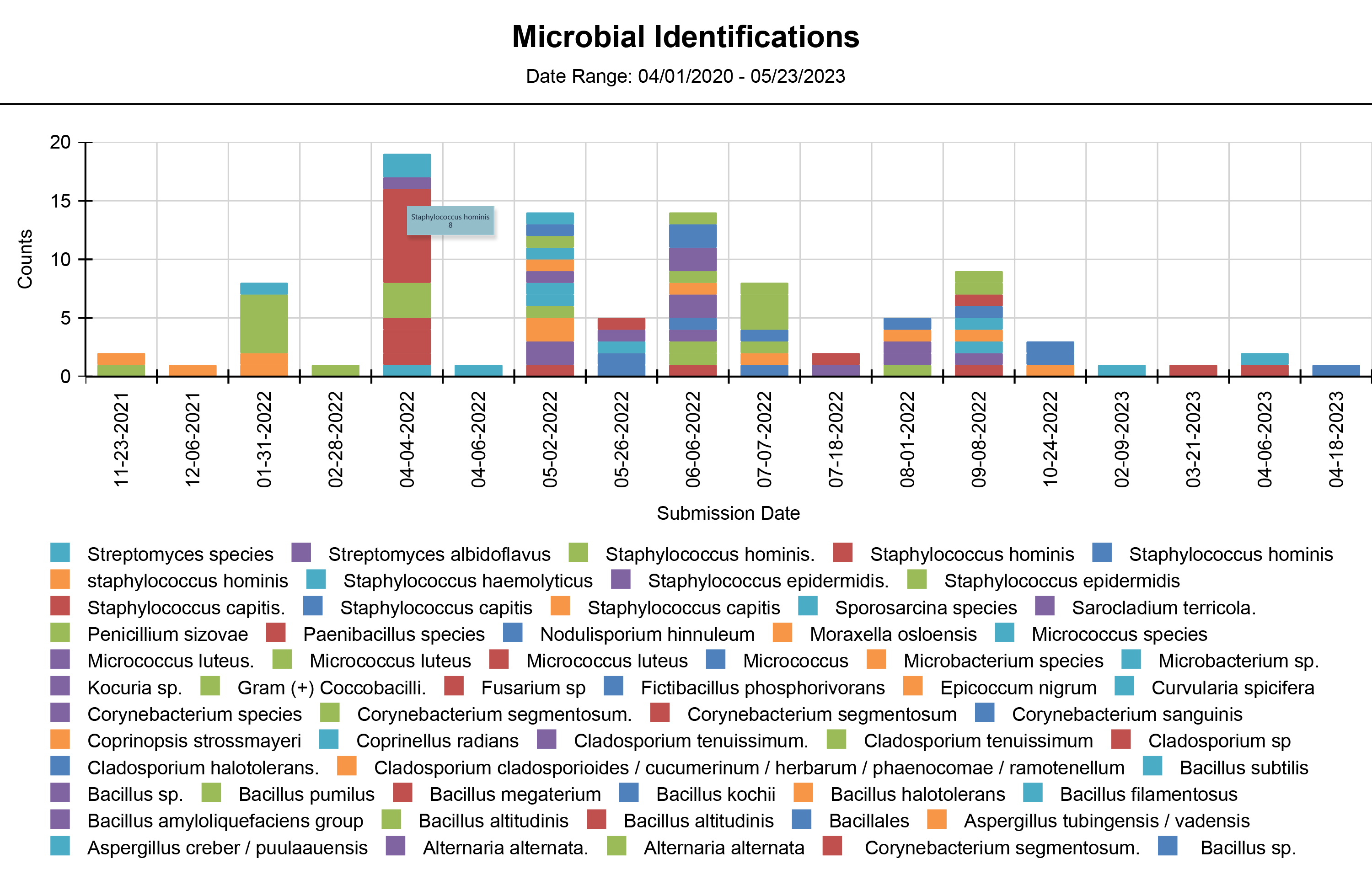
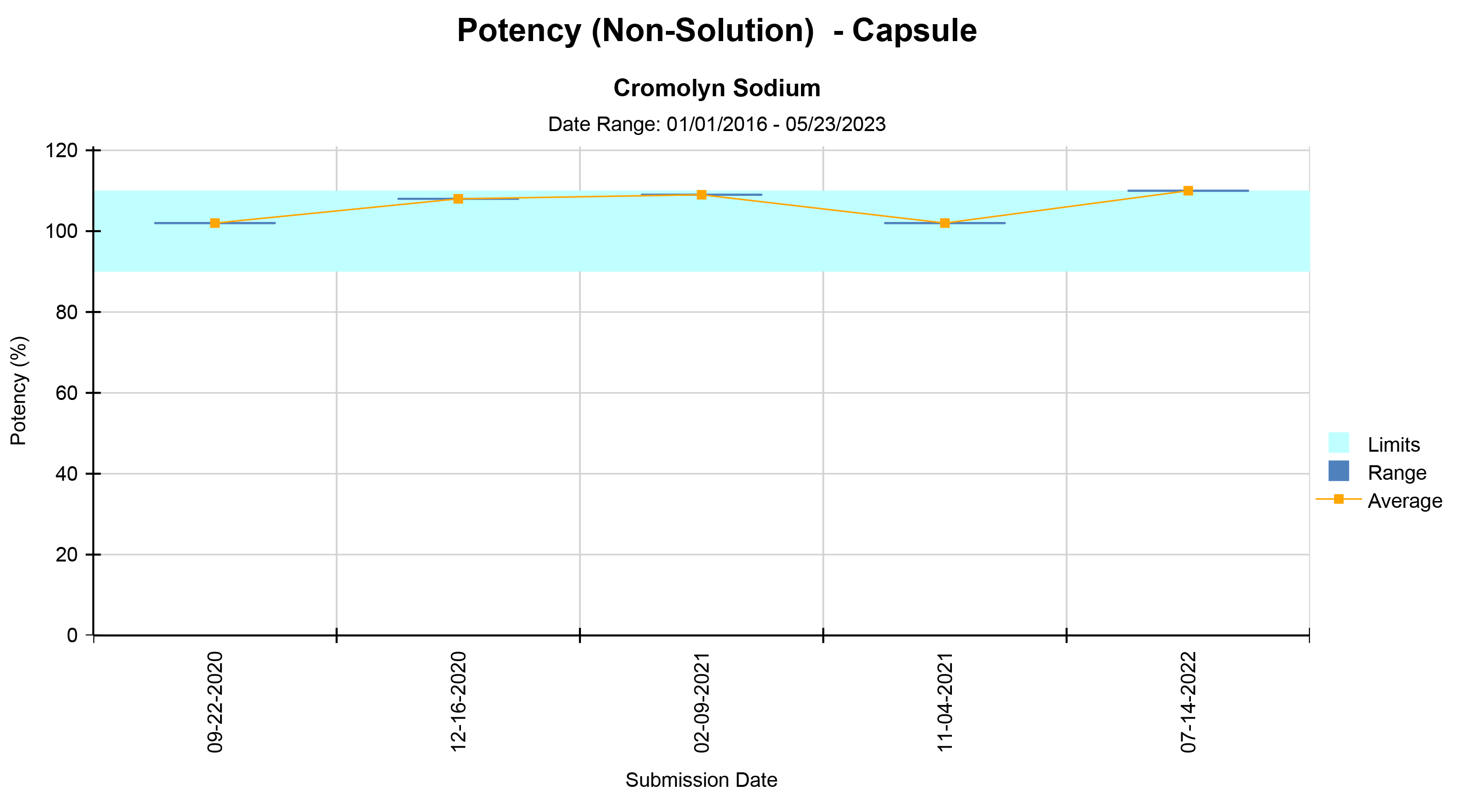
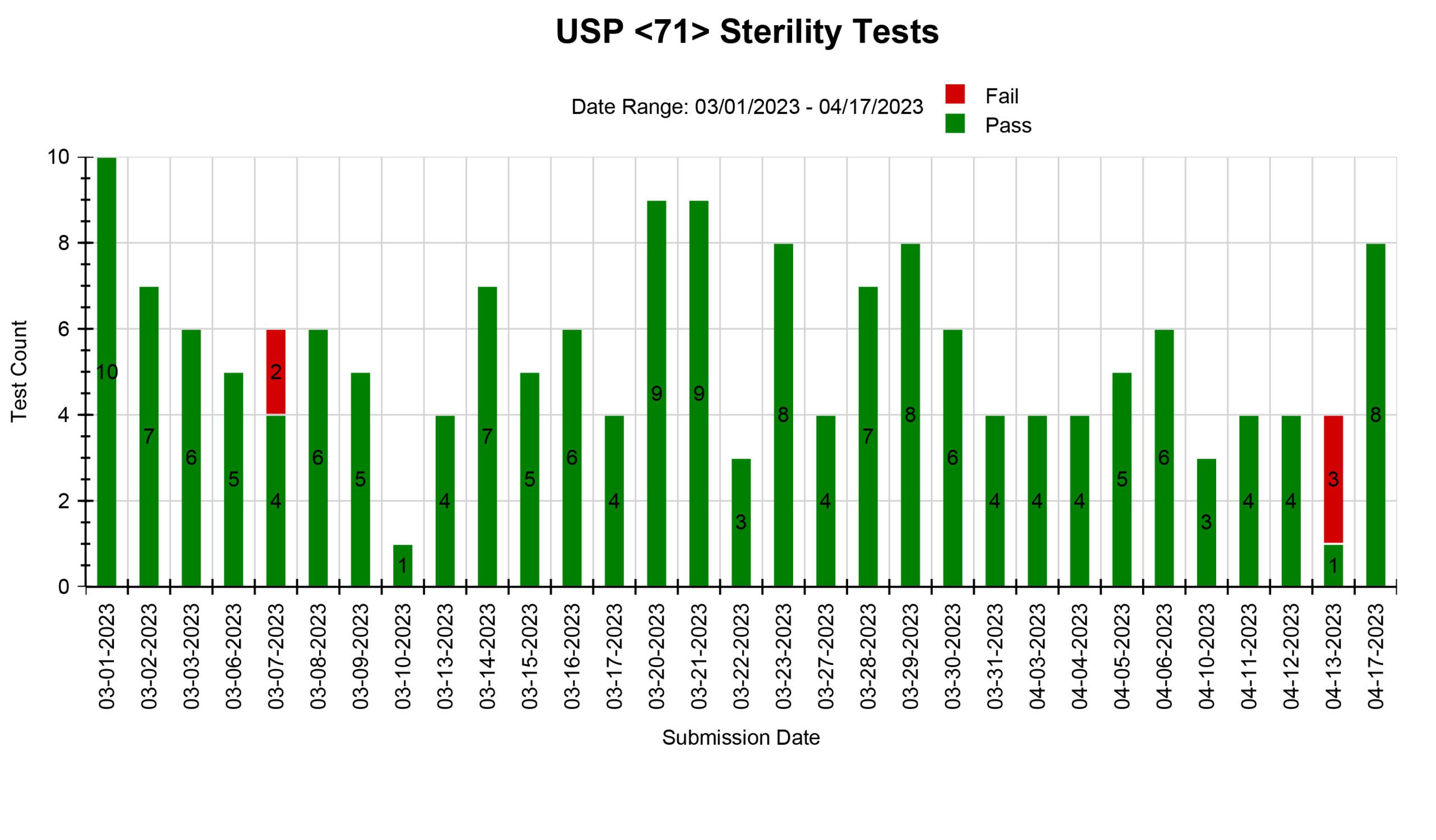
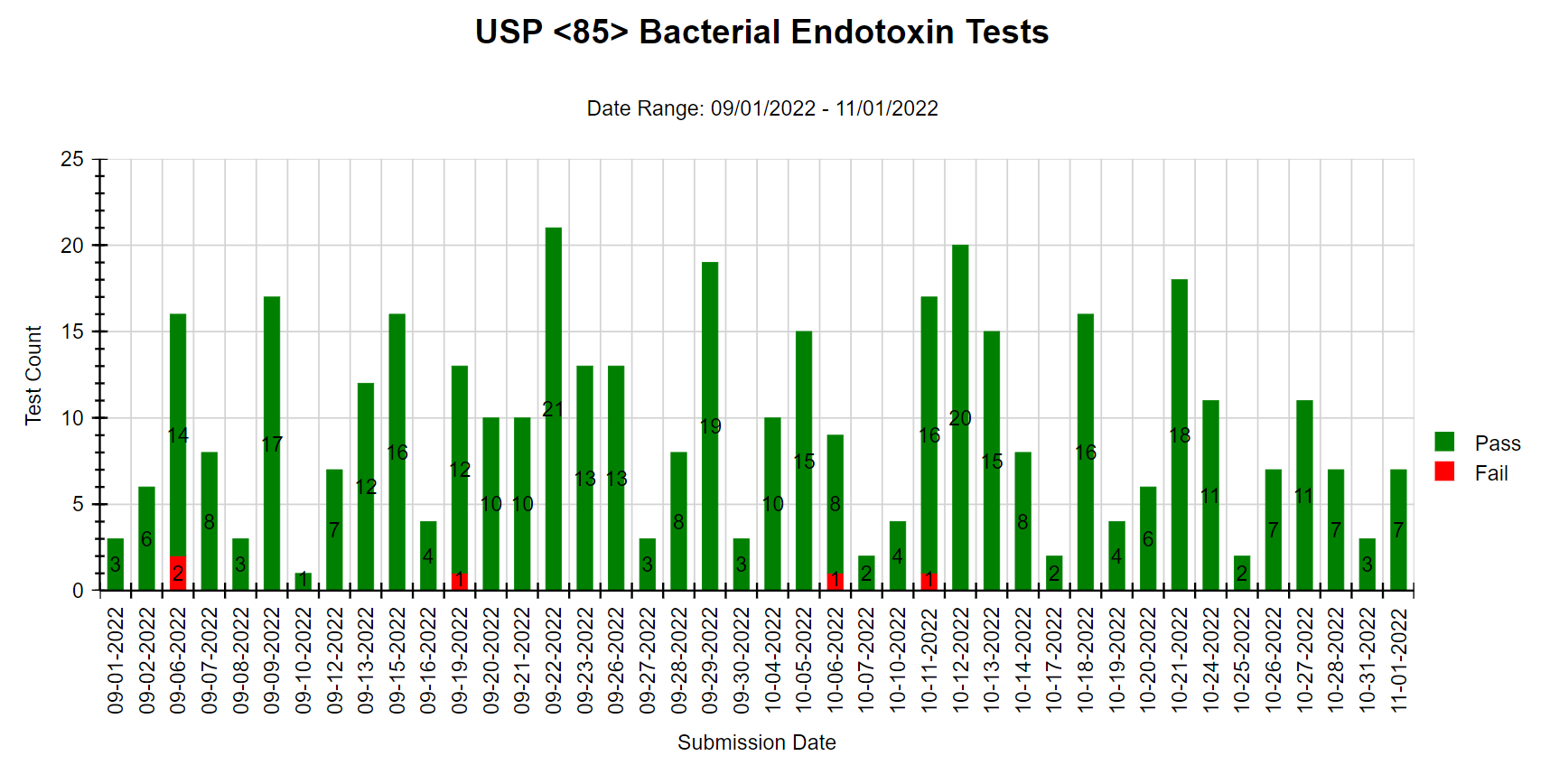
Shield Analysis Informational Series
An informational series created to assist you in understanding and navigating EagleTrax’s Shield Analysis statistical process control charts. Save this page to your favorites for quick access to new Shield Analysis videos, how-to instructions, pdfs, and related content.
Creating a Sample Submission for Environmental Monitoring Sampling
- Begin by filling out the “General Information” section with the following details:
- Sample name: Enter “EM” or “Environmental Monitoring” followed by the type of sampling (ex. air, surface, etc.) and plate used (ex. 65mm). This field is mandatory and will be displayed in the trend report.
- Compounders: Enter the name of the person who took the sampling.
- Compounding date: Enter the date when the sampling was conducted.
- Lot #: Enter the location and ISO class of where the sampling was conducted. This field is mandatory and will be displayed in the trend report.
- Dosage From: Enter “plate”.
- Total submission amount: Enter “1” (1 plate per submission).
- Sample Description: Use this field to provide any specific information important for your facility regarding this plate. Note that it will not be displayed in the report.
- Storage conditions: Select “room temperature.”
- Move to the “Add Analyte” section:
- Analyte: Select “Plate.”
- Concentration: Enter “1”
- Unit of Measure: Select “None.“
- Proceed to the “Tests” section:
- Department: Select “Microbiology.”
- Category: Select “Bacteriology.”
- Test: Depending on the test needed, incubation, plate enumeration, & microbial identification can all be performed at Eagle. If incubation and counting of CFU are not required, microbial identification will only need to be selected.
Note: Ensure that you have filled all the required fields in the “General Information” and “Analyte” sections before selecting the requested tests in the “Tests” section; otherwise, the options will populate as “inactive.”
If you need to submit several plates, you can utilize the “Copy” option:
- Go to the Find Submission section.
- Find the submission you want to copy and click on the copy icon next to the submission number.
- Check the boxes for the sections you want to copy to the next submission (General Information, Submission Analytes, Submission Tests, Submission Documents).
- After copying, only certain fields of the general information section will need to be completed for the new submission.
OUR LABORATORY
Eagle’s cutting-edge 35,000-square-foot facility and laboratory are equipped with advanced technologies and specialized segregated laboratory spaces to meet the diverse needs of our clients. This behind-the-scenes video provides you with an opportunity to witness testing while touring our laboratory.

