Thank you for reading this post, don't forget to subscribe!
ABOUT US
Eagle is a U.S. Food and Drug Administration (FDA) and Drug Enforcement Administration (DEA) registered A2LA ISO 17025 accredited testing/calibration (Cert.# 3980.01 and 3980.02) laboratory founded in 2003 that provides analytical chemistry and microbiological testing, consultation services, and other technical services for pharmaceutical manufacturers, pharmacies, medical device companies, and other highly regulated industries, to support their compliance needs.
TOGETHER WE SOAR | A Message From Eagle’s President & CEO

Our desire, dedication, and discipline to ensure that patient safety is at the forefront of everything we do and our holistic approach to resolving your compliance needs make Eagle a truly unique organization.
Eagle was established two decades ago to meet the regulatory needs and requirements of the pharmaceutical industry. During that time, Eagle grew and morphed into a full-fledged analytical chemistry and microbiological testing laboratory and consulting firm. In the coming decade, Eagle will continue building greater capabilities so that we can meet new market demands.
We are committed to providing your organization with the tools and knowledge to operate and maintain compliance within a highly regulated environment. Our science and pharmaceutical experts, state-of-the-art equipment, dynamic systems, and advanced procedures allow us to offer superior customized services.
Our desire, dedication, and discipline to ensure that patient safety is at the forefront of everything we do and our holistic approach to resolving your compliance needs make Eagle a truly unique organization.
We thank you for allowing us to assist you. We pride ourselves on being a valuable resource for you and look forward to facing future challenges together.
Sincerely,
Ross A. Caputo
EAGLE’S MISSION
Guide and educate pharmacists and healthcare professionals to utilize fact-based decision-making to create science-based solutions.
OUR VISION
Research and develop innovative scientific approaches to solve healthcare challenges.
TAKING FLIGHT WITH EAGLE, OUR HISTORY
Since 2003, Eagle has helped provide organizations with the tools necessary to operate and maintain compliance in the highly regulated CGMP industry. Through continued learning and innovation, Eagle strives to serve you better, so you may be at your best for those who depend on you.
Leadership and Management Team, We Are Here To Help
As the leadership and management of EAGLE, we are committed to having a quality mindset and a continuous drive to achieve better patient safety for all. We understand the gravity of our work and embrace the responsibility of it.

Ross Caputo, Ph.D.
President & Chief Executive Officer

David Hussong, Ph.D.
Chief Technical Officer

Lisa Johnson, BS
Vice President of Marketing & Business Development

Robert Byrne, Ph.D.
Vice President of Scientific Affairs

Jay Patel, Ph.D.
Vice President of Analytical Sciences

Miguel Hernandez, MS
Director of Quality Assurance/Quality Control

Jacqueline Esqueda, PharmD
Senior Business Development
OUR COMMITMENT TO YOU
It is our commitment to provide your organization with the tools to operate and maintain compliance within a highly regulated environment. In support of this commitment, Eagle is now home to professionals spanning the science and pharmaceutical industry, including experts in chemistry, microbiology, engineering, CGMP, quality control, and quality assurance.
OUR EXPERTS
Our scientific experts excel at helping sterile and non-sterile facilities identify risks, challenges, opportunities, and implement cost-effective, science-based solutions to resolve your needs. With our guidance, you will feel confident with your compliance.

OUR LABORATORIES
Eagle’s cutting-edge 35,000-square-foot facility and laboratory are equipped with advanced technologies and specialized segregated laboratory spaces to meet the diverse needs of our clients. This behind-the-scenes video provides you with an opportunity to witness testing while touring our laboratory.

Your Future Awaits
Each of us has unique talents, but we share a common desire to enhance the lives of those around us. If you seek to join a dynamic and inventive company, Eagle could be the ideal fit for you.
 August 2003
August 2003  May 2004
May 2004 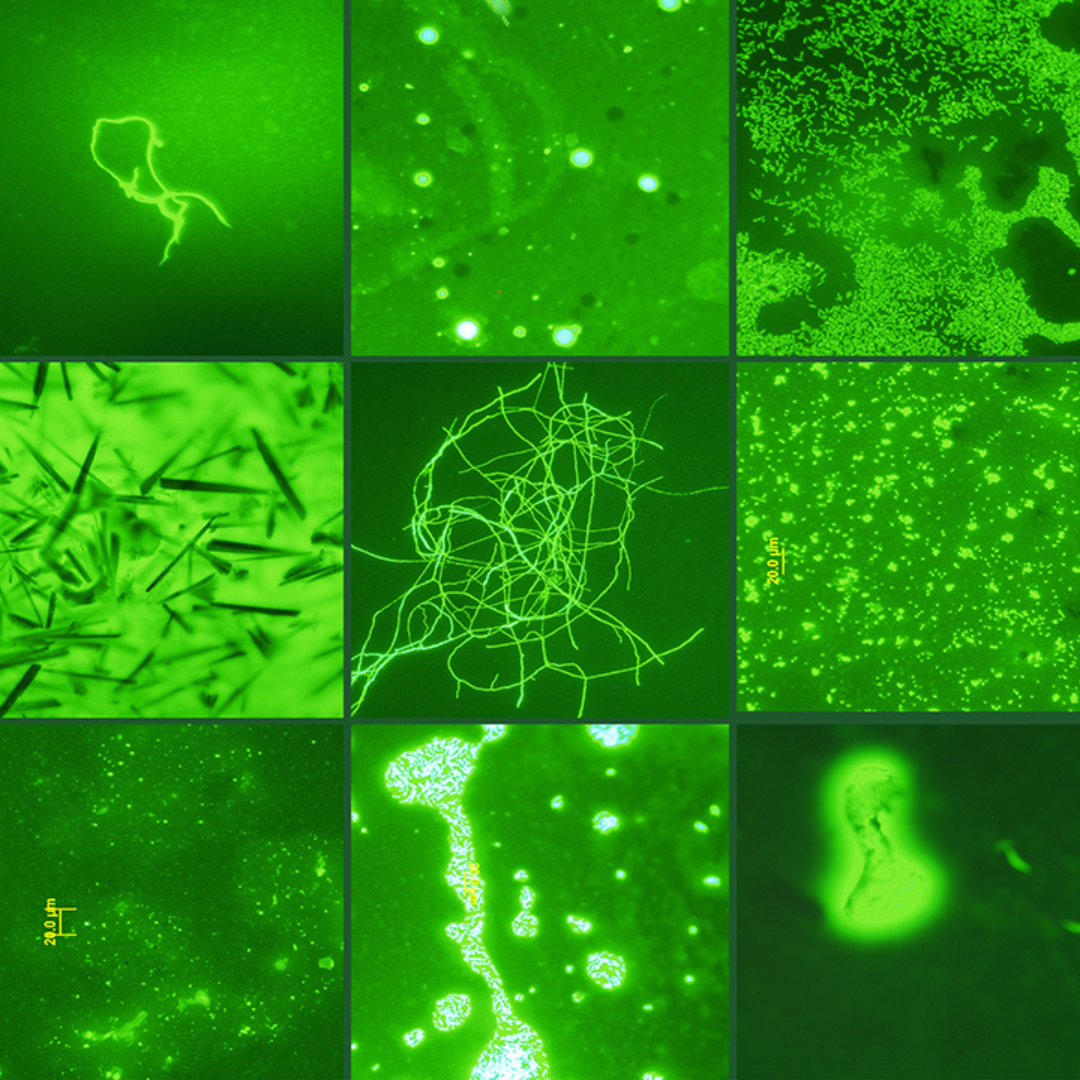 May 2004
May 2004  2010
2010  November 2013
November 2013  August 2015
August 2015  April 2016
April 2016  May 2016
May 2016  July 2017
July 2017  November 2017
November 2017  January 2018
January 2018  April 2019
April 2019  June 2019
June 2019  July 2019
July 2019  2020
2020  January 2021
January 2021  October 2021
October 2021  September & November 2021
September & November 2021  October 2022
October 2022 