Industry Leading Solutions Pharmaceutical BioTech Pharmacy
With over 200 years of combined experience in the FDA-regulated Pharmaceutical Industry, Eagle assists facilities mitigate risk by providing compliance and safety solutions, backed by science. Our interdisciplinary team, in addition to routine Laboratory Testing, Eagle is uniquely positioned to provide services in Research and Development, Consulting, Calibration, Certification, Qualification, Facility Design, Validation, and Compliance Auditing.
Rapid Sterility Testing 1-2 Business Days! Faster Way To Comply
The ScanRDI® is a unique alternative technology that enables Eagle to deliver sterility testing results in 1 or 2 business days, allowing sterile compounding facilities to release product faster than when utilizing the compendial USP <71> method. ScanRDI® has revolutionized rapid microbial detection and its combination of speed and sensitivity remains unrivaled to this day. Additionally, ScanRDI® fulfills the method suitability requirement as outlined in USP <1223>.
Taking Flight With Eagle Eagle Eagle
It is our commitment to provide your organization with the tools to operate and maintain compliance within a highly regulated environment. In support of this commitment, Eagle is now home to professionals spanning the science and pharmaceutical industry, including experts in chemistry, microbiology, engineering, CGMP, quality control, and quality assurance.
Eagle's Mission
Guide and educate pharmacists and healthcare professionals to utilize fact-based decision-making to create science-based solutions.
Our Vision
Research and develop innovative scientific approaches to solve healthcare challenges.
Commitment To You
We are committed to having a quality mindset and a continuous drive to achieve better patient safety for all.


Commitment to You Customer Service Unparalleled Value Quality Assurance
It is our unwavering commitment to provide your organization with the tools to operate and maintain compliance within a highly regulated environment. In support of this commitment, Eagle is now home to professionals spanning the science and pharmaceutical industry, including experts in chemistry, microbiology, engineering, CGMP, quality control, and quality assurance.
Compliance with Regulatory Standards
Trusted by the Industry: Customer Satisfaction
Introducing Real-Time Sample Updates Results & more!
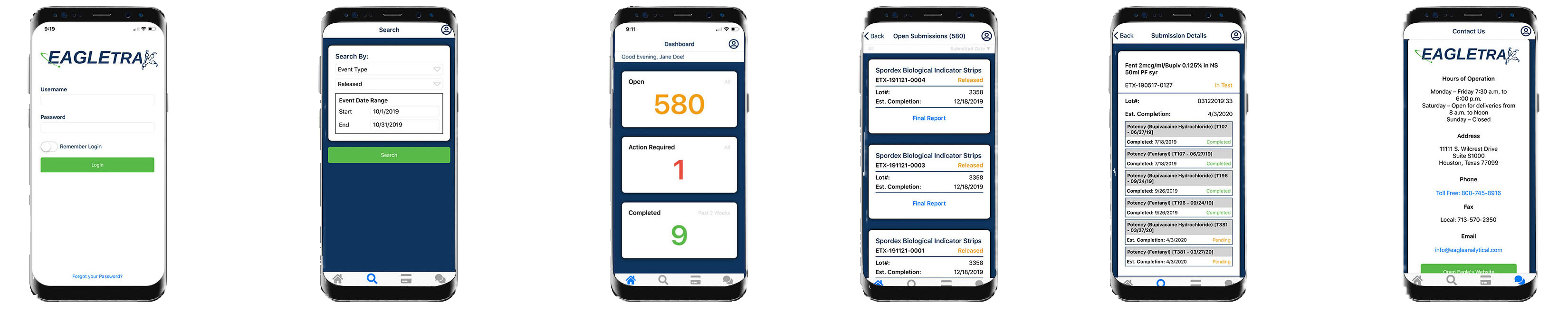 Our EagleTrax system empowers you to efficiently manage your samples, offering the flexibility to perform many essential tasks on the go through the mobile interface. While some advanced features are optimized for the desktop version, the system ensures seamless access and functionality across both platforms to meet your needs.
Our EagleTrax system empowers you to efficiently manage your samples, offering the flexibility to perform many essential tasks on the go through the mobile interface. While some advanced features are optimized for the desktop version, the system ensures seamless access and functionality across both platforms to meet your needs.
Search Your Submissions
Real-time Sample Updates
Process Control Charts
Live Sample Results
View & Print Reports
Pay Bills Online
Trusted by More Than 5k Clients Around The World The World
Our clients consistently praise Eagle for its exceptional service, reliability, and dedication to exceeding expectations.
 "
"
Sherry Eghtesadi
PharmD
"Our experience with Eagle has gone beyond getting reliable and accurate data on our daily testing needs. Eagle’s highly trained team provides quick solutions to our problems through their quality consultative services and courteous care. Being a customer of Eagle is a privilege. It’s a confident decision to add value to our businesses."

 "
"
Jim Hrncir
Rph
"With the health of my patients and the health of my business so dependent on my analytical lab, the choice is obvious. Eagle has been there for me with professional training and advice, cutting-edge technology/accuracy, and even in emergency situational response. It seems apparent that Eagle’s business model is focused on the success of its clients."

 "
"
Danny Carrero
Rph, President
“Eagle has become an integral part of ensuring we deliver safe compounds and maintain the quality that allows us to have trust among pet owners and veterinarians. We utilize a triple-check system to test our compounded medications and rely on their superb service and expertise on a regular basis. The level of competence exhibited by the representatives I deal with gives me confidence as a pharmacist, and I’m delighted to be doing business with Eagle.”

Emil Haldey
“My experience with Eagle has been nothing short of exceptional! Their professionals have years of experience and are very knowledgeable about the industry. I am especially impressed with the high level advice from Ross and the consulting team, as well as their guidance relating to organizational compliance and business growth. I highly recommend Eagle for any of your testing or consulting needs!”
Need Help Finding The Necessary Science-Based Solution?
Eagle provides analytical chemistry and microbiological testing, consultation services, and other technical services for pharmaceutical manufacturers, pharmacies, medical device companies, and other highly regulated industries, to support their compliance needs.
Send us a Message Industry ExpertsTesting ServicesConsulting Solutions
Contact the Eagle Client Care Team to discuss your operation’s specific needs!
Reflecting on Two Decades of Innovation and Growth
As part of our 20th-anniversary celebration, we've invited team members from different departments to share their thoughts and feelings about working at Eagle.











