Sterility Testing
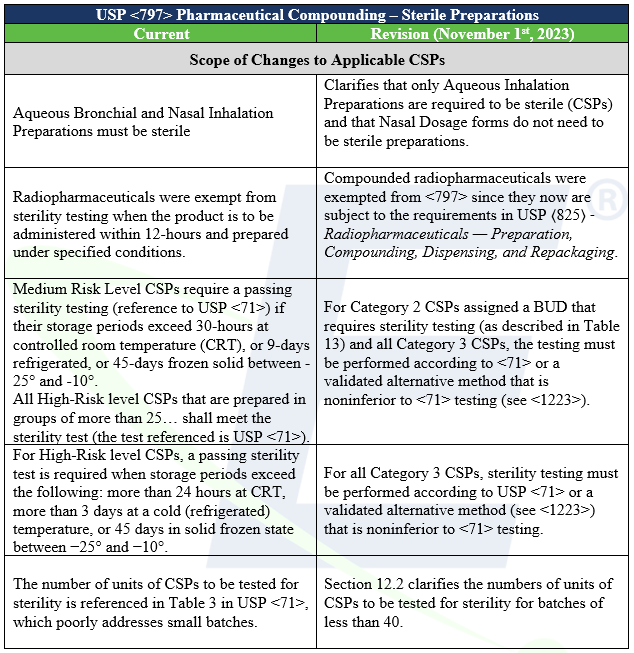
The updated revisions to USP Chapters <795> Pharmaceutical Compounding – Nonsterile Preparations and <797> Pharmaceutical Compounding – Sterile Preparations became official on November 1st, 2023 and the conditions established are enforceable by regulatory bodies. Both chapters underwent significant changes making it important for compounders to understand the quality control expectations to ensure they remain compliant. USP Chapter <795> deals only with non-sterile preparations and will not be discussed here. USP Chapter <797> is specific to Compounded Sterile Preparations, so sterility testing is relevant only to this discussion.
Major Changes from the Older Chapter Related to Testing Preparations for the Quality Attribute of Sterility
The quality attribute “sterile” attribute describes a condition that “cannot be applied to actual items labeled as sterile because of irresolvable limitations in testing” (USP <1211> Sterility Assurance). These irresolvable limitations are statistical and biological. While the attribute “sterile” applies to a complete absence of living organisms (an absolute condition), there is no test that can detect all living organisms, and the complete absence of living organisms could only be tested if the entire production of the preparation were tested (and thus destroyed). Then as a practical issue, only a portion of the production may be tested, and only a portion of contaminants may be detected by any test methods. For this reason, Chapter <797> places a great deal of emphasis on practices that provide “Quality Assurance” that will improve “Sterility Assurance” to the final Compounded Sterile Preparation (CSP).
A significant clarification to the revised text refers to the need for passing USP <71> “Sterility Test” to text that included alternative test methods that were validated and not inferior to USP <71>. This clarification conforms with USP “General Notices” section 6.30 which states:
“An alternative method or procedure is defined as any method or procedure other than the compendial method or procedure for the article in question. The alternative method or procedure must be fully validated (see Validation of Compendial Procedures <1225>) and must produce comparable results to the compendial method or procedure within allowable limits established on a case-by-case basis. Alternative methods or procedures can be developed for any one of several reasons not limited to simplification of sample preparation, enhanced precision and accuracy, improved (shortened) run time, or being better suited to automation than the compendial method or procedure. Only those results obtained by the methods and procedures given in the compendia are conclusive.”
Let Our Experience Work For You
Cutting-Edge Our Laboratories
Eagle’s cutting-edge 80,000-square-foot facility and laboratory are equipped with advanced technologies and specialized segregated laboratory spaces to meet the diverse needs of our clients. This behind-the-scenes video provides you with an opportunity to witness testing while touring our laboratory.

Get Started Today
Got Sterility Testing Needs?
Call the Eagle Client Care Team at (800) 745-8916 to discuss your operation’s specific needs and answer any questions!