EAGLE IS MORE THAN TESTING, LET OUR KNOWLEDGE WORK FOR YOU
GAP ANALYSIS AUDIT | COMPLIANCE SOLUTIONS & CONSULTING | CALIBRATIONS & CERTIFICATIONS | TESTING | & MORE
Water Activity
Water Activity Revisions
Thank you for reading this post, don't forget to subscribe!
A notable change introduced in the new USP <795> Pharmaceutical Compounding – Nonsterile Preparations chapter, is the use of water activity as a tool for establishing the beyond-use dates (BUDs) for compounded nonsterile preparations (CNSPs). It should be emphasized, however, that water activity is not referenced in the revised USP <797> Pharmaceutical Compounding – Sterile Preparations chapter and should not be utilized to establish the BUD of a compounded sterile preparation (CSP).
In the past, pharmacists were tasked with combing through multiple sources of literature and previous studies to determine an appropriate BUD for a specific formulation. However, revisions to USP <795> that will take effect on November 1, 2023, introduce the use of a dosage forms’ water activity as the basis for establishing a BUD for compounded nonsterile preparations. This approach has simplified the process of assigning BUDs by taking the guesswork out of the equation and eliminating the need to review multiple sources; ultimately saving the pharmacist time and effort.
The new practice of using water activity as a tool for establishing the BUD of a CNSP is described in Section 10.3 of the to-be-official USP <795> chapter. Water activity testing in accordance with USP <922> is not required but is highly recommended to support the established BUD of the CNSP. Water activity testing should be considered in cases where there is an absence of a USP-NF Compounded Preparation Monograph and/or CNSP-specific stability information.
Water activity is the ratio between the standard water vapor pressure and the water pressure reflected in a sample. There is a general limit on water activity depending on the type of product you have. If the value of water activity exceeds this limit, the moist environment may support or enhance microbial growth. It is important to note that water activity is not the same as water content. Water content refers to the amount of water in a sample, whereas water activity refers to how much water is available for microbial growth.
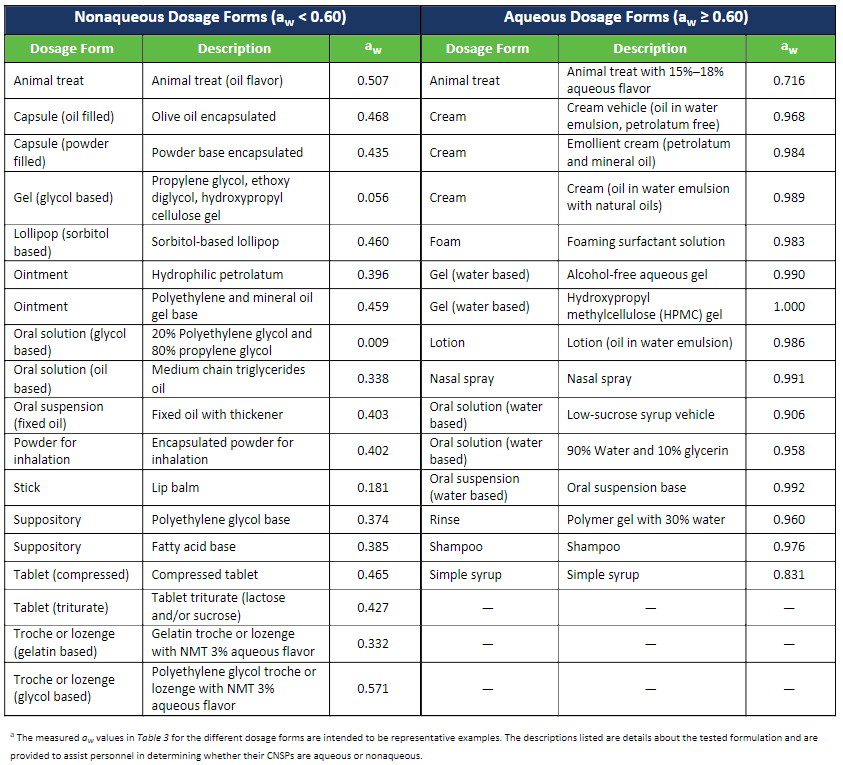
To assist pharmacists in determining their BUDs, the revised USP <795> chapter has introduced a table outlining the water activity for common CNSP dosage forms.
Table 1, Water Activity of Common Compounded Nonsterile Dosage Forms, which correlates to Table 3 in the to-be-official USP <795> chapter, lists the water activity for common nonsterile, aqueous and nonquaeous dosage forms.
Table 1. Water Activity of Common Compounded Nonsterile Dosage Forms
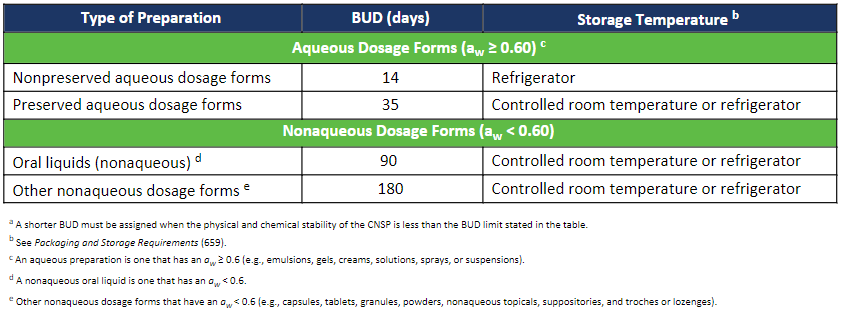
Table 2, BUD Limit by Type of Preparation in the Absence of a USP−NF Compounded Preparation Monograph or CNSP-Specific Stability Information, which correlates to Table 4 in the to-be-official USP <795> chapter, outlines the BUD limits by type of preparation, aqueous or nonaqueous, storage condition, and water activity.
Table 2. BUD Limit by Type of Preparation in the Absence of a USP-NF Compounded Preparation Monograph or CNSP-Specific Stability Information ᵃ
It is important to note that the tabulated water activity for the listed dosage forms are only estimates and some CNSPs may not be chemically stable through the defined BUD limits. It is the responsibility of the designated person to assure that the established BUD for the CNSP is appropriate for its water activity and chemical stability. Additionally, BUDs should not exceed the expiration date of any of the ingredients in the CNSP.
If the BUD of an aqueous CNSP is extended beyond the limits in Table 2, the suitability of the antimicrobial agent for preventing the growth of bacteria, yeast, and mold contamination must be determined through USP <51> Antimicrobial Effective (AME) Testing. This testing is necessary for formulations that exceed a water activity of 0.60, as they are at potential risk of supporting microbial growth during the compounding process, handling, and under certain storage conditions. AME test results are limited to a specific CNSP formulation in its final container-closure system. Pharmacists may rely on published AME results, but only if the CNSP formulation, ingredients, and packaging materials are equivalent.
How to Prepare
As requirements change, it is important that pharmacists review their master formulation records to ensure that BUDs in the absence of formulation and container-closure-specific stability studies are congruent with the revised chapter. Additionally, to assure that the process for assigning BUDs is consistent from CNSP to CNSP, standard operating procedures should be revised to reflect the upcoming revisions.
To learn more about water activity testing, antimicrobial effectiveness testing, or stability studies, contact a member of our Client Care Team today.
OUR LABORATORIES
Eagle’s cutting-edge 35,000-square-foot facility and laboratory are equipped with advanced technologies and specialized segregated laboratory spaces to meet the diverse needs of our clients. This behind-the-scenes video provides you with an opportunity to witness testing while touring our laboratory.