EAGLE IS MORE THAN TESTING, LET OUR KNOWLEDGE WORK FOR YOU
GAP ANALYSIS AUDIT | COMPLIANCE SOLUTIONS & CONSULTING | CALIBRATIONS & CERTIFICATIONS | TESTING | & MORE
Thank you for reading this post, don't forget to subscribe!
Cleanroom Certification
Despite revisions to the sterile compounding chapter, USP <797> that became official on November 1, 2023, the requirements for cleanroom certification remain consistent with the 2008 standards.
Cleanroom certification shall be performed prior to initiating sterile operations and must be repeated at least every six months and/or after any changes to the classified area including construction, replacement, or relocation of any primary engineering control (PEC), and any reconfiguration of the room and major equipment.
Classified areas and engineering controls must be certified to meet the requirements for the following test:
Airflow Testing
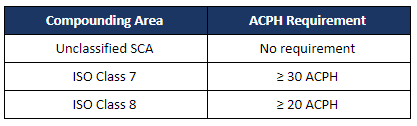
Airflow testing includes a measure of air changes per hour (ACPH) to determine the acceptability of the air velocity and the room air exchange rate to ensure that the appropriate quality of air is maintained during dynamic operating conditions.
The total ACPH must be documented on the certification and must include the air changes contributed by the heating, ventilation, and air conditioning (HVAC) system and the primary engineering controls (PEC). At least 15 of the total air changes must come from the HVAC system. The minimum ACPH with respect to ISO classified spaces are:
Differential Pressure Testing
Differential pressures are measured between adjacent rooms of classified spaces to ensure that air flows from areas of higher air quality to areas of lower air quality and to verify the direction of airflow as required for the type of compounding operations (hazardous versus non-hazardous). For positive pressure rooms, a minimum differential pressure of 0.020-inch water column (w.c.) is required for adjacent ISO-classified areas and between the anteroom and the unclassified space. For hazardous drug compounding, a negative differential pressure of 0.010 to 0.030-inch w.c. is required for adjacent areas.
HEPA Filter Integrity Testing
HEPA filters must be leak tested after installation and with every certification. A known concentration of aerosol is introduced upstream of the HEPA filter and compared to the downstream concentration to evaluate for leaks. Detected leaks may be patched as long as they do not exceed 3% of the filter area.
Total Airborne Particle Count Testing
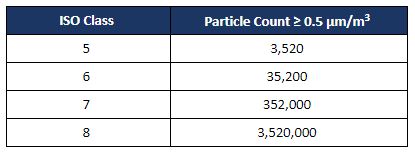
Total airborne particle sampling must be performed in all ISO-classified spaces including the PECs under dynamic operations to ensure that levels remain within acceptable limits of air cleanliness during compounding operations. The total number of personnel during routine operation, which can include the certifier, must be present in the space during certification and documented on the certification report.
The number of sampling locations is defined by ISO 14644-1 and is based on the size of the room. Sampling locations should be spread out evenly and represent roughly the same area within the room. Contact a member of our team to learn more.
Limits for the number of particles ≥ 0.5 µm/m3 with respect to ISO classification:
Dynamic Airflow Pattern Testing (Smoke Studies)
Airflow pattern testing, commonly referred to as smoke studies, is used to demonstrate unidirectional airflow of the PEC and a sweeping action over and away from the direct compounding area. Smoke studies must be performed under dynamic operating conditions and should encompass all aseptic processes, including associated equipment and materials, maximum number of personnel, and the highest complexity of compounding performed at the facility during routine operation.
Smoke studies must be video recorded and should capture airflow patterns at rest (with associated equipment and materials in place) to evaluate whether equipment placement contributes to adverse air currents such as turbulence, reflux, and stagnant air. At rest conditions should be evaluated prior to the execution of the dynamic smoke study to ensure that adverse air currents are appropriately addressed. The smoke study videos must include the following information:
- Date
- Facility name and site location
- Equipment ID number
- Operational state (as-build, at-rest, dynamic/operational)
Airflow pattern studies should be accompanied by a report summarizing the results of the study and readily available for regulatory inspectors. It is best practice to capture airflow pattern footage in one continuous video, however, if videos are edited, the raw footage should be available for review.
Smoke studies must be repeated any time equipment placement and materials are changed to assure that first air to the direct compounding area is maintained.
Viable Environmental Monitoring
Viable environmental monitoring is essential in evaluating the microbial state of control of the sterile compounding facility and is typically performed in tandem with cleanroom certification. Environmental monitoring (EM) for viable microorganisms is comprised of air and surface sampling.
Viable air and surface sampling locations should be defined by the compounding facility and specified in the standard operating procedures (SOPs). Sampling locations should be selected based on a risk assessment and must include all classified areas and all PECs. Ideal sampling locations include those with the highest activity, pose the highest risk to the final compounded sterile preparation (CSP), and high touch areas. Sampling locations may include:
- Staging and storage areas, especially for materials transferred into the PEC
- Equipment located within the PEC
- Pass-throughs
- Restricted-access barrier systems (RABS)
- High-touch areas (pumps, keyboards, light switches, door handles, door plates, etc.)
- High-traffic areas
- Near PECs, pass-throughs, and sinks
- Identified areas of stagnant or turbulent air (outside of the PEC)
Action levels are defined in USP <797> Pharmaceutical Compounding – Sterile Preparations and are as follows:
Certification reports must be reviewed by the designated person(s) to assure that all requirements have been met. The number of personnel present during the dynamic total particle-count tests, smoke studies, and viable environmental monitoring must be documented in the report. Results that are out-of-specification must be corrected and investigated to evaluate their impact on compounded sterile preparations produced within the space. Controls should be implemented to evaluate the adequacy of corrective actions and prevent recurrence of events.
We recommend scheduling cleanroom certification in a timely manner and planning accordingly to avoid workflow disruptions and delays in production.
To learn more about cleanroom certification, and any of the requirements therein, or to schedule your certification, contact a member of our Client Care Team today.
OUR OPERATIONS DEPARTMENT
Calibration, certification, qualification, and facility design review services are offered by Eagle’s Operations Department. The operations team consents of dedicated engineering and logistics professionals who are committed to providing expert and excellent care to our customers.

OUR LABORATORIES
Eagle’s cutting-edge 35,000-square-foot facility and laboratory are equipped with advanced technologies and specialized segregated laboratory spaces to meet the diverse needs of our clients. This behind-the-scenes video provides you with an opportunity to witness testing while touring our laboratory.